Amphibian
Declines
- Declines of populations of amphibians around the world have
been a prominent issue in conservation biology for more than a
decade (Hayes and Jennings 1986; Barinaga 1990; Bradford 1991;
Carey 1993; Fellers and Drost 1993; Kagarise Sherman and Morton
1993; Blaustein 1994a, b; Drost and Fellers 1996; Lannoo 1998;
Lips 1998; Knapp and Matthews 2000; Reaser 2000; Alexander and
Eischeid 2001; Carey et al. 2001; Young et al. 2001).
- Quantifying declines and understanding their ecological relevance
is challenging because there have been few long-term studies of
populations.
- We need to obtain new baseline
data for populations and subsequently monitor them over time.
- Data from such studies help us establish statuses and trends
and understand the context of fluctuations in populations over
time.
- Environmental stressors that can cause declines include loss
of habitat, disease, pollutants, climate change, ultrDecember 29, 2010ation by introduced species, among others
(Bradford 1991; Griffiths and Beebee 1992; Blaustein et al. 1994;
Bradford et al. 1994; Pounds and Crump 1994; Carey and Bryant
1995; Kupferberg 1997; Adams 2000; Carey 2000; Knapp and Matthews
2000; Alford et al. 2001; Davidson et al. 2001; Gillespie 2001;
Kiesecker et al. 2001; Pounds 2001; Blaustein and Kiesecker 2002;
Hayes et al. 2003).
- The relevance and intensity of environmental stressors vary
in space and time and tolerances to those stressors vary with
individuals, populations, and species (Griffiths and Beebee 1992;
Alford et al. 2001; Blaustein and Kiesecker 2002).
- Evaluating the relevance and intensity of stressors requires
measuring and monitoring appropriate environmental variables;
understanding tolerances requires manipulative experiments to
determine dose-response relationships and thresholds.
- Given inherent fluctuations in populations, quantifying declines
can take years (Griffiths and Beebee 1992; Pechmann and Wilbur
1994; Blaustein and Kiesecker 2002).
- Even when a decline has been described, demonstrating its relation
to specific stressors at requisite multiple scales can be daunting
(Corn and Fogleman 1984; Griffiths and Beebee 1992; Davidson et
al. 2001; Young et al. 2001; Blaustein and Kiesecker 2002).
- Scientific approaches to these challenges require accumulating
complementary evidence in support or rejection of specific hypotheses
pertaining to the status of a population and detrimental effects
of specific stressors on that population.
- Given the complexity of these issues, the cumulative lines of
evidence necessary to unequivocally link a decline to specific
causes can prove elusive, especially for declines that occurred
in the past.
Declines in the
Midwest Region of ARMI
- Some populations of amphibians in the Midwest have declined
and face the same threats as populations in other locations (Lannoo
et al. 1994; Lannoo 1998).
- Dramatic declines have not been reported from this region on
a scale similar to those reported from the western United States
(e.g., Corn and Fogleman 1984; Bradford 1991; Carey 1993; Kagarise
Sherman and Morton 1993; Bradford et al. 1994; Drost and Fellers
1996; Lannoo 1998; Knapp and Matthews 2000; Reaser 2000; Carey
et al. 2001).
- The lack of reports of dramatic declines in this region could reflect a combination of less actual recent declines,
relative stability among populations currently, and a lack of
appropriate data to describe declines adequately.
- Many of the declines in the western states have been observed
in mountainous regions where relatively long-term, large-scale
studies have been conducted by visual encounter surveys (Carey
1993; Kagarise Sherman and Morton 1993; Blaustein et al. 1994;
Bradford et al. 1994; Kiesecker and Blaustein 1995; Drost and
Fellers 1996; Knapp and Matthews 2000).
- Few comparable studies have been reported from the UMR of ARMI.
- We do not understand historical variation or current status
well enough (Pechmann and Wilbur 1994) to describe current population
levels or their trajectories for most populations in the UMR of
ARMI (Lannoo 1998).
- Populations of northern leopard frogs (Rana pipiens)
and Blanchard’s cricket frogs (Acris crepitans
blanchardi) declined over the past several decades
(e.g., Corn and Fogleman, 1984; Lannoo et al. 1994; Hay
1998; Lannoo 1998; Moriarity 1998).
|
- We do not know the locations of or trends in the distributions
and abundances of most remaining populations well enough
to evaluate their relative stabilities.
- Various state and conservation organizations have designated
other species of conservation concern due to declines or
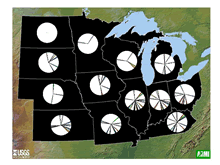
threats (Table 1;
Figure 6), but similar uncertainties
exist regarding the statuses of most of those populations.
|
|
|
Potential Causes
of Declines in the Midwest Region of ARMI
Deformities
- Relatively high frequencies of deformed frogs at some locations
have gained the most attention regarding amphibians in the UMR
of ARMI (Reaser and Johnson 1997; Lannoo 1998; Gardiner and Hoppe
1999; Burkhart et al. 2000; Helgen et al. 2000; Hopkins et al.
2000; Meteyer et al. 2000; Johnson et al. 2001, 2002).
- We know that deformities are widespread (North American
Reporting Center for Amphibian Malformations [NARCAM] 2003;
Blaustein and Johnson 2003) perhaps to some extent because
surveys have been conducted more extensively and intensively
in recent years.
- Whereas high frequencies of deformities have occurred
at specific locations, frequencies often are inconsistent
at and across sites, and high frequencies do not appear
to be widespread (NARCAM 2003; D. Green pers. com.), including
in the UMR (Converse et al. 2000; NARCAM 2003; R. Cole pers.
com.; D. Green pers. com.; M. Knutson pers. com.; D. Sutherland
pers. com.).
|

Deformed northern leopard frog ( Rana pipiens) |
- Deformities often are associated with infections by the parasitic
trematode Ribeiroia (Johnson et al. 2001, 2002; Blaustein
and Johnson 2003). Johnson and Chase (2004) reported
recently that the snails in which early life stages of Ribeiroia
live before they invade tadpoles were more abundant in wetlands
with high levels of nitrogen and phosphorous. However, lines
of evidence from the laboratory and field have not been conclusive
regarding the combinations of factors that can act on populations
to cause deformities and to what extent, if any, deformities are
linked to declines (Reaser and Johnson 1997; Ankley et al. 1998;
Burkhart et al. 1998; Gardiner and Hoppe 1999; Dournon et al.
1998; Helgen et al. 2000; Hopkins et al. 2000; Sower et al. 2000;
Gillilland et al. 2001; Johnson et al. 2001, 2002; Blaustein and
Johnson 2003).
- Deformities can result from biotic and abiotic factors and get
considerable attention in the scientific and popular presses,
but little evidence so far suggests that they are sufficiently
frequent or consistent to cause declines.
- The potential for high frequencies of deformities to indicate
some form of ecological instability because of environmental stress
(Blaustein and Johnson 2003) could portend future declines of
populations of amphibians and warrants monitoring frequencies
and types of deformities.
- We continue to survey and monitor animals for deformities and
send appropriate specimens to Dr. David Green at the USGS National
Wildlife Health Center for analyses, but we do not plan to devote
our efforts to studying deformities more intensively at this time.
- Our efforts could change in future years with improved understanding
of any roles deformities play in declines of populations.
Habitat loss,
agricultural practices, and climate change
Habitat
loss, including fragmentation
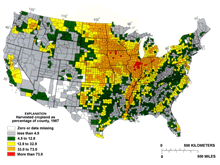
- Enormous quantities of wetlands and upland habitat have
been lost to urban development and alteration for agriculture
across the Midwest (Lannoo 1998)
(Figure 7).
|
|
|

Fragmented agricultural landscape
|
- Such loss of habitat undoubtedly has eliminated or reduced
the distribution and abundance of many populations of amphibians
and continues to threaten many others (Hager 1998; Lannoo
1998; Knutson et al. 1999; Kolozsvary and Swihart 1999;
Lehtinen et al. 1999; Semlitsch 2000; 2002; Gaggiotti 2003;
Hazell 2003; Houlahan and Findlay 2003; Weyrauch and Grubb
2004).
|
- Meaningful analyses of the statuses of populations of amphibians
in the UMR of ARMI cannot be conducted without understanding the
threats posed by the extensive habitat loss and fragmentation
in this region.
- For example, a population and its habitat might be or become
fragmented because of habitat destruction or alteration.
Such changes also could cause the population to become isolated
from that of other populations because of the loss of habitat
in between. Thus, the population might exist today, but
it could go extinct due to loss of too much local habitat or to
being cutoff from immigrants from nearby populations (Bradford
et al. 1993; Lehtinen et al. 1999; Semlitsch 2000; Ovaskainen
and Hanski 2003).
- We are working with researchers from Iowa State University and
the USGS EROS Data Center to develop methods to assess the connectedness
of populations across the landscape and to study relationships
between habitat loss, connectedness, and vulnerability of populations
to extinction.
Agricultural practices
- In addition to destruction and fragmentation of habitats,
agricultural use of pesticides and fertilizers poses potential
threats to populations by lethal and sublethal mechanisms
(Lannoo 1998; Bishop et al. 1999; Marco and Blaustein 1999;
Bridges et al. 2000; Bridges and Semlitsch 2000; Semlitsch
et al. 2000; Davidson et al. 2001; Marco et al. 2001; Sparling
et al. 2001; Kiesecker 2002; Relyea 2003, 2004).
|
- Hayes et al. (2002, 2003) and Carr et al. (2003) published
evidence recently that atrazine can induce abnormal gonadal
development in frogs, as was suggested earlier by Reeder
et al. (1998).
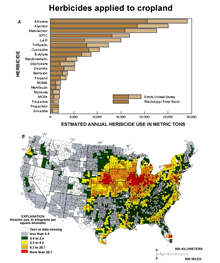
- Atrazine is the most heavily used herbicide in the Midwest
and the United States (Figure
8 ). Thus, these reports are cause for concern
with respect to potential past and present effects on populations
of amphibians (Hayes et al. 2002; 2003).
- We are measuring levels of atrazine and similar compounds
in the water at breeding sites we are monitoring.
We also are collecting frogs from some of these sites to
analyze them for gonadal abnormalities.
|
|
Climate change
- Concern among scientists over human-induced changes in global
climate continues to increase (e.g., Hughes 2000; McCarty 2001;
Pounds 2001; Walther et al. 2002; Flanagan et al. 2003; O’Reilly
2003; Trenberth 2003; Thomas et al. 2004).
- Evidence suggests that global warming is associated with intensification
of the hydrologic cycle, leading to greater extremes of floods
and droughts in many regions, including much of the Mississippi
River Basin (Houghton et al. 1996; Karl and Knight 1998; Knox
2000; Groisman et al. 2001; Milly et al. 2002).
- Rainfall increased in the Upper Mississippi River Basin over
the last 30 years, driving increased runoff and transport of nitrate
(and presumably pesticides) into tributaries from agricultural
lands (Randall and Mulla 2001; Donner et al. 2002; Donner and
Kucharik 2003) .
- In addition, higher spring temperatures in the Upper Mississippi
River Basin may have directly influenced land use, resulting in
earlier planting of crops (Kucharik in prep.) and applications
of fertilizer (Donner et al. in review) and pesticides.
- Amphibians are sensitive to climate-driven variation in hydroperiod,
humidity, and temperature (Pounds and Crump 1994; Blaustein et
al. 2001).
- Droughts have been associated with declines of populations of
amphibians in the Sierra Nevada Mountains (Kagarise Sherman and
Morton 1993).
- Changes in climate have been associated with declines in Costa
Rica (Pounds and Crump 1994), breeding phenology in Great Britain
(Beebee 1995), and the incidence of UVB-induced mortality in the
Cascade Mountains of Oregon (Kiesecker et al. 2001).
- Cold temperatures and insufficient precipitation can result
in frozen embryos and breeding sites can dry before metamorphosis
can occur (Sadinski pers. obs.).
- Insufficient snowfall can result in soils and water freezing
to depths that could kill or harm overwintering amphibians (Irwin
et al. 1999).
- Higher frequencies of such conditions could cause substantially
reduced recruitment in populations of amphibians than has occurred
in the recent past.
- Climate change, among all of the potential universal stressors,
has a uniquely powerful potential to affect populations of amphibians,
either by directional changes in temperature and precipitation
or higher frequencies of extreme weather.
- We are working to establish sites where we can monitor relationships
between the dynamics of populations and climate over time.
Literature Cited
Adams, M. J. 2000. Pond permanence and the effects of exotic
vertebrates on anurans. Ecological Applications 10(2):559-568.
Alexander, M. A. and J. K. Eischeid. 2001. Climate variability in
regions of amphibian declines. Conservation Biology 15(4):930-942.
Alford, R. A., P. M. Dixon, and J. H. K. Pechmann. 2001. Global
amphibian population declines. Nature 412(6846):499-500.
Ankley, G., E. Mihaich, R. Stahl, D. Tillitt, T. Colborn, S. McMaster,
R. Miller, J. Bantle, P. Campbell, N. Denslow, R. Dickerson, L.
Folmar, M. Fry, J. Giesy, L. E. Gray, and et al. 1998. Overview
of a workshop on screening methods for detecting potential (anti-)
estrogenic/androgenic chemicals in wildlife. Environmental Toxicology
and Chemistry 17(1):68-87.
Barinaga, M. 1990. Where have all the froggies gone? Science 247:1033-1034.
Beebee, T. J. C. 1995. Amphibian breeding and climate. Nature 374(6519):219-220.
Bishop, C. A., N. A. Mahony, J. Struger, P. Ng, and K. E. Pettit.
1999 . Anuran development, density and diversity in relation to
agricultural activity in the Holland River watershed, Ontario, Canada
(1990-1992). Environmental Monitoring and Assessment 57(1):21-43.
Blaustein, A. R. 1994a. Chicken Little of Nero's Fiddle? A perspective
on declining amphibian populations. Herpetologica 50(1):85-97.
Blaustein, A. R. 1994b. Amphibians in a bad light. Natural History
103(10):32-39.
Blaustein, A. R., P. D. Hoffman, D. G. Hokit, J. M. Kiesecker, S.
C. Walls, and J. B. Hays. 1994. UV repair and resistance to solar
UV-B in amphibian eggs: A link to population declines? Proceedings
of the National Academy of Sciences, USA 91(5):1791-1795.
Blaustein, A. R. and P. T. J. Johnson. 2003. Explaining frog deformities.
Scientific American 288(2):60-65.
Blaustein, A. R. and J. M. Kiesecker. 2002. Complexity in conservation:
lessons from the global decline of amphibian populations. Ecology
Letters 5(4):597-608.
Blaustein, A. R., E. L. Wildy, L. K. Belden, and A. Hatch. 2001.
Influence of abiotic and biotic factors on amphibians in ephemeral
ponds with special reference to long-toed salamanders (Ambystoma
macrodactylum). Israel Journal of Zoology 47(4):333-345.
Bradford, D. F. 1991. Mass mortality and extinction in a high-elevation
population of Rana muscosa. Journal of Herpetology 25(2):174-177.
Bradford, D. F., D. M. Graber, and F. Tabatabai. 1994. Population
declines of the native frog, Rana muscosa, in Sequoia and
Kings Canyon national parks, California. Southwestern Naturalist
39(4):323-327.
Bradford, D. F., F. Tabatabai, and D. M. Graber. 1993. Isolation
of remaining populations of the native frog, Rana muscosa,
by introduced fishes in Sequoia and Kings Canyon national parks,
California. Conservation Biology 7(4):882-888.
Bridges, C. M. 2000. Long-term
effects of pesticide exposure at various life stages of the Southern
Leopard Frog (Rana sphenocephala). Archives of Environmental
Contamination and Toxicology 39(1):91-96
Bridges, C. M. and R. D. Semlitsch. 2000. Variation in pesticide
tolerance of tadpoles among and within species of Ranidae and patterns
of amphibian decline. Conservation Biology 14(5):1490-1499.
Burkhart, J. G., G. Ankley, H. Bell, H. Carpenter, D. Fort, D. Gardiner,
H. Gardner, R. Hale , J. C. Helgen, P. Jepson, D. Johnson, M. Lannoo,
D. Lee, J. Lary, and et al. 2000. Strategies for Assessing the Implications
of Malformed Frogs for Environmental Health. Environmental Health
Perspectives 108(1):83-90.
Burkhart, J. G., J. C. Helgen, D. J. Fort, K. Gallagher, D. Bowers,
T. L. Propst, M. Gernes, J. Magner, M. D. Shelby, and G. Lucier.
1998. Induction of mortality and malformation in Xenopus laevis
embryos by water sources associated with field frog deformities.
Environmental Health Perspectives 106(12):841-848.
Carey, C. 1993. Hypothesis concerning the causes of the disappearance
of boreal toads from the mountains of Colorado. Conservation Biology
7(2):355-362.
Carey, C. 2000. Infectious disease and worldwide declines of amphibian
populations, with comments on emerging diseases in coral reef organisms
and in humans. Environmental Health Perspectives Supplement 108(1):143-150.
Carey, C. and C. J. Bryant. 1995. Possible interrelations among
environmental toxicants, amphibian development, and decline of amphibian
populations. Environmental Health Perspectives 103(4):13-17.
Carey, C., W. R. Heyer, J. Wilkinson, R. A. Alford, J. W. Arntzen,
T. Halliday, L. Hungerford, K. R. Lips, E. M. Middleton, S. A. Orchard,
and A. S. Rand. 2001. Amphibian declines and environmental change:
Use of remote-sensing data to identify environmental correlates.
Conservation Biology 15(4):903-913.
Carr, J. A., A. Gentles, E. E. Smith, W. L. Goleman, L. J. Urquidi,
K. Thuett, R. J. Kendall, J. P. Giesy, T. S. Gross, and K. R. Solomon.
2003. Response of larval Xenopus laevis to atrazine: assessment
of growth, metamorphosis, and gonadal and laryngeal morphology.
Environmental Toxicology and Chemistry 22(2):396-405.
Converse, K. A., J. Mattsson, and L. Eaton-Poole. 2000. Field surveys
of midwestern and northeastern Fish and Wildlife Service lands for
the presence of abnormal frogs and toads. Journal of the Iowa Academy
of Science 107(3):160-167.
Corn, P. S. and J. C. Fogleman. 1984. Extinction of montane populations
of the northern leopard frog (Rana pipiens) in Colorado.
Journal of Herpetology 18(2):147-152.
Davidson, C., H. B. Shaffer, and M. R. Jennings. 2001. Declines
of the California red-legged frog: climate, UV-B, habitat, and pesticides
hypotheses. Ecological Applications 11(2):464-479.
Donner, S. D., M. T. Coe, J. D. Lenters, T. E. Twine, and J. A.
Foley. 2002. Modeling the impact of hydrological changes on nitrate
transport in the Mississippi River Basin from 1955-1994. Global
Biogeochemical Cycles 16:1-19.
Donner, S. D. and C. J. Kucharik. 2003. Evaluating the impacts of
land management and climate variability on crop production and nitrate
export across the Upper Mississippi Basin. Global Biogeochemical
Cycles 17(3):1085-1102.
Donner, S. D., C. J. Kucharik, and J. A. Foley. The impact of changing
land use practices on nitrate export by the Mississippi River. In
review.
Dournon, C., A. Bautz, H. Membre, M. Lauthier, and A. Collenot.
1998. Expression of hind limb abnormalities under rearing temperature
effects during the larval development of the salamander Pleurodeles
waltl (urodele amphibian). Development Growth and Differentiation
40(5):555-565.
Drost, C. A. and G. M. Fellers. 1996. Collapse of a regional frog
fauna in the Yosemite area of the California Sierra Nevada, USA.
Conservation Biology 10(2):414-425.
Fellers, G. M. and C. A. Drost. 1993. Disappearance of the Cascades
frog Rana cascadae at the southern end of its range, California,
USA. Biological Conservation 65(2):177-181.
Flanagan, K. M., E. McCauley, F. Wrona, and T. Prowse. 2003. Climate
change: the potential for latitudinal effects on algal biomass in
aquatic ecosystems. Canadian Journal of Fisheries and Aquatic Sciences
60(6):639-639.
Gaggiotti, O. E. 2003. Genetic threats to population persistence.
Annales Zoologici Fennici 40(2):155-168.
Gardiner, D. M. and D. M. Hoppe. 1999. Environmentally induced limb
malformations in mink frogs (Rana septentrionalis). The
Journal of Experimental Zoology 284(2):207-216.
Gillespie, G. R. 2001. The role of introduced trout in the decline
of the spotted tree frog (Litoria spenceri) in south-eastern
Australia. Biological Conservation 100(2):187-198.
Gillilland, C. D., C. L. Summer, M. G. Gillilland, K. Kannan, D.
L. Villeneuve, K. K. Coady, P. Muzzall, C. Mehne, and J. P. Giesy.
2001. Organochlorine insecticides, polychlorinated biphenyls, and
metals in water, sediment, and green frogs from southwestern Michigan.
Chemosphere 44(3):327-339.
Griffiths, R. and T. Beebee. 1992. Decline and fall of the amphibians.
New Scientist 134(1827):25-29.
Groisman, P. Y., R. W. Knight, and T. R. Karl. 2001. Heavy precipitation
and high stream flow in the contiguous United States: trends in
the 20th century. Bulletin of the American Meteorological Society
82:219-246.
Hager, H. A. 1998. Area-sensitivity of reptiles and amphibians:
Are there indicator species for habitat fragmentation? Ecoscience
5(2):139-147.
Hay, R. 1998. Blanchard's cricket frogs in Wisconsin: A status report.
Pages 79-82 in Lannoo, M. J. editor. Status and conservation of
midwestern amphibians. Lannoo, M. J. Ed. University of Iowa Press,
Iowa City.
Hayes, M. P., and M. R. Jennings. 1986. Decline of ranid frog species
in western North America: Are bullfrogs (Rana catesbeiana)
responsible? Journal of Herpetology 20(4):490-509.
Hayes, T. B., A. Collins, M. Lee, M. Mendoza, N. Noriega, A. A.
Stuart, and A. Vonk. 2002.
Hermaphroditic, demasculinized
frogs after exposure to the herbicide atrazine at low ecologically
relevant doses. Proceedings of the National Academy of Sciences,
USA 99(8):5476-5480.
Hayes, T. B., K. Haston, M. Tsui, A. Hoang, C. Haeffele, and A.
Vonk. 2003. Atrazine-induced hermaphroditism at 0.1 ppb in American
leopard frogs (Rana pipiens): laboratory and field evidence.
Environmental Health Perspectives 111(4):568-575.
Hayes, T. B., K. Haston,
M. Tsui, A. Hoang, C. Haeffele, and A. Vonk. 2002 . Feminization
of male frogs in the wild. Nature 419(6910):895-896.
Hazell, D. 2003. Frog ecology in modified Australian landscapes:
a review. Wildlife Research 30(3):193-205.
Helgen, J. C., M. C. Gernes, S. M. Kersten, J. W. Chirhart, J. T.
Canfield, D. Bowers, J.
Haferman, R. G. McKinnell, and D. M. Hoppe. 2000. Field investigations
of malformed frogs in Minnesota, 1993-97. Journal of the Iowa Academy
of Science 107(3):96-112.
Hopkins, W. A., J. Congdon, and J. K. Ray. 2000. Incidence and impact
of axial malformations in larval bullfrogs (Rana catesbeiana)
developing in sites polluted by a coal-burning power plant. Environmental
Toxicology and Chemistry 19(4):862-868.
Houlahan, J. E. and C. S. Findlay. 2003. The effects of adjacent
land use on wetland amphibian species richness and community composition.
Canadian Journal of Fisheries and Aquatic Sciences 60(9):1078-1094.
Houghton, J. T., L. G. Meira Filho, B. A. Callander, N. Harris,
A. Kattenberg, and K. Maskell editors. 1996. Climate change 1995.
the science of climate change. Cambridge University Press Boston,
MA.
Hughes, L. 2000. Biological consequences of global warming: Is the
signal already apparent? Trends in Ecology and Evolution 15(2):56-61.
Irwin, J. R., J. P. Costanzo,
and R. E. Lee Jr. 1999. Terrestrial hibernation in the northern
cricket frog, Acris crepitans. Canadian Journal of Zoology
1999(8)1240-1246.
Johnson, P. T. J. and
J. M. Chase. 2004. Parasites in the food web:
linking amphibian malformations and aquatic eutrophication.
Ecology Letters 7(7):521.
Johnson, P. T. J., K.
B. Lunde, R. W. Haight, J. Bowerman, and A. R. Blaustein. 2001.
Ribeiroia ondatrae (Trematoda: Digenea) infection induces
severe limb malformations in western toads (Bufo boreas).
Canadian Journal of Zoology/Revue Canadien de Zoologie 79(3):370-379.
Johnson, P. T. J., K. B. Lunde, E. M. Thurman, E. G. Ritchie, S.
N. Wray, D. R. Sutherland, J. M. Kapfer, T. J. Frest, J. Bowerman,
and A. R. Blaustein. 2002. Parasite (Ribeiroia ondatrae)
infection linked to amphibian malformations in the western United
States. Ecological Monographs 72(2):151-168.
Kagarise Sherman, C. and M. L. Morton. 1993. Population declines
of Yosemite toads in the eastern Sierra Nevada of California. Journal
of Herpetology 27(2):186-198.
Karl, T. R. and R. W. Knight. 1998. Secular trends of precipitation
amount, frequency, and intensity in the United States. Bulletin
of the American Meteorological Society 79:231-241.
Kiesecker, J. M. 2002. Synergism between trematode infection and
pesticide exposure: A link to amphibian limb deformities in nature?
Proceedings of the National Academy of Sciences, USA 99(15):9900-9904.
Kiesecker, J. M. and A. R. Blaustein. 1995. Synergism between UV-B
radiation and a pathogen magnifies amphibian embryo mortality in
nature. Proceedings of the National Academy of Sciences, USA 92(24):11049-11052.
Kiesecker, J. M., A. R. Blaustein, and L. K. Belden. 2001. Complex
causes of amphibian population declines. Nature 410(6829):681-684.
Knapp, R. A. and K. R. Matthews. 2000. Non-native fish introductions
and the decline of the mountain yellow-legged frog from within protected
areas. Conservation Biology 14(2):428-438.
Knox, J. C. 2000. Sensitivity of modern and holocene floods to climate
change. Quaternary Science Reviews 19:439-457.
Knutson, M. G., J. R. Sauer, D. A. Olsen, M. J. Mossman, L. M. Hemesath,
and M. J. Lannoo. 1999. Effects of landscape composition and wetland
fragmentation on frog and toad abundance and species richness in
Iowa and Wisconsin, USA. Conservation Biology 13(6):1437-1446.
Kolozsvary, M. B., and R. K. Swihart. 1999. Habitat fragmentation
and the distribution of amphibians: patch and landscape correlates
in farmland. Canadian Journal of Zoology/Revue Canadien de Zoologie
77(8):1288-1299.
Kupferberg, S. J. 1997. Bullfrog (Rana catesbeiana) invasion
of a California river: The role of larval competition. Ecology 78(6):1736-1751.
Lannoo, M. J. 1998. Status and conservation of midwestern amphibians.
University of Iowa Press, Iowa City.
Lannoo, M. J., K. Lang, T. Waltz, and G. S. Phillips. 1994. An altered
amphibian assemblage: Dickinson County, Iowa, 70 years after Frank
Blanchard's survey. American Midland Naturalist 131(2):311-319.
Lehtinen, R. M., S. M. Galatowitsch, and J. R. Tester. 1999. Consequences
of habitat loss and fragmentation for wetland amphibian assemblages.
Wetlands 19(1):1-12.
Lips, K. R. 1998. Decline
of a tropical montane amphibian fauna. Conservation Biology 12(1):106-117.
Marco, A. and A. R. Blaustein. 1999. The effects of nitrite on behavior
and metamorphosis in cascades frogs (Rana cascadae). Environmental
Toxicology and Chemistry 18(5):946-949.
Marco, A., D. Cash, L. K. Belden, and A. R. Blaustein. 2001. Sensitivity
to urea fertilization in three amphibian species. Archives of Environmental
Contamination and Toxicology 40(3):406-409.
McCarty, J. P. 2001. Ecological consequences of recent climate change.
Conservation Biology 15(2):320-331.
Meteyer, C. U., I. K. Loeffler, J. F. Fallon, K. A. Converse, E.
Green, J. C. Helgen, S. Kersten, R. Levey, L. Eaton-Poole, and J.
G. Burkhart. 2000. Hind limb malformations in free-living northern
leopard frogs (Rana pipiens) from Maine, Minnesota, and
Vermont suggest multiple etiologies. Teratology 62(3):151-171.
Milly, P. C. D., R. T. Wetherald, K. A. Dunne, and T. L. Delworth.
2002. Increasing risk of great floods in a changing climate. Nature
415:514-517.
Moriarty, J. J. 1998. Status of amphibians in Minnesota. Pages 166-167
in Lannoo, M. J. editor. Status and conservation of midwestern
amphibians. University of Iowa Press, Iowa City.
North American Reporting Center for Amphibian Malformations. 2003.
http://frogweb.nbii.gov/narcam/.
O'Reilly, C. M., S. R. Alin, P. D. Plisnier, A. S. Cohen, and B.
A. McKee. 2003. Climate change decreases aquatic ecosystem productivity
of Lake Tanganyika, Africa. Nature 424(6950):766-768.
Ovaskainen, O. and I. Hanski. 2003. Extinction threshold in metapopulation
models. Annales Zoologici Fennici 40(2):81-97.
Pechmann, J. H. K. and H. M. Wilbur. 1994. Putting declining amphibian
populations in perspective natural fluctuations and human impacts.
Herpetologica 50(1):65-84.
Pounds, J. A. 2001. Climate and amphibian declines. Nature 410(6829):639-640.
Pounds, J. A. and M. L. Crump. 1994. Amphibian declines and climate
disturbance: The case of the golden toad and the harlequin frog.
Conservation Biology 8(1):72-85.
Randall, G. W. and D. J. Mulla. 2001. Nitrate nitrogen in surface
waters as influenced by climatic conditions and agricultural practices.
Journal of Environmental Quality 30:337-344.
Reaser, J. K. and Johnson, P. T. 1997. Amphibian abnormalities:
a review. Science 284:802-804.
Reaser, J. K. 2000. Amphibian declines: an issue overview. Washington,
DC. http://www.frogweb.gov/declines.pdf
Reeder, A. L., G. L. Foley, D. K. Nichols, L. B. Hansan, B. Wikoff,
S. Faeh, M. Eisold, B. Wheeler, R. Warner, J. E. Murphy, and V.
R. Beasley. 1998. Forms and prevalence of intersexuality and effects
of environmental contaminants on sexuality in cricket frogs (Acris
crepitans). Environmental Health Perspectives 106(5):261-266.
Relyea, R. A. 2003. Predator
cues and pesticides: a double dose of danger for amphibians.
Ecological Applications 13(6):1515-1521.
Relyea, R. A. 2004. Synergistic
impacts of malathion and predatory stress on six species of North
American tadpoles. Environmental Toxicology and Chemistry
23(4):1080-1084.
Semlitsch, R., C. Bridges, and A. Welch. 2000. Genetic variation
and a fitness tradeoff in the tolerance of gray treefrog (Hyla
versicolor) tadpoles to the insecticide carbaryl. Oecologia
125(2):179-185.
Semlitsch, R. D. 2000. Principles for management of aquatic-breeding
amphibians. Journal of Wildlife Management 64(3):615-631.
Semlitsch, R. D. 2002. Critical elements for biologically based
recovery plans of aquatic-breeding amphibians. Conservation Biology
16(3):619-629.
Sower, S. A., K. L. Reed, and K. J. Babbitt. 2000. Limb malformations
and abnormal sex hormone concentrations in frogs. Environmental
Health Perspectives 108(11):1085-1090.
Sparling, D. W., G. M. Fellers, and L. L. McConnell. 2001. Pesticides
and amphibian population declines in California, USA. Environmental
Toxicology and Chemistry 20(7):1591-1595.
Thomas, C. D., A. Cameron, R. E. Green, M. Bakkenes, L. J. Beaumont,
Y. C. Collingham, B. F. N. Erasmus, M. F. de Siqueira, A. Grainger,
L. Hannah, L. Hughes, B. Huntley, A. S. Can Jaarsveld, G. F. Midgley,
L. Miles, M. A. Ortega-Huerta, A. T. Peterson, O. L. Phillips, and
S. E. Williams. 2004. Extinction risk from climate change. Nature
427(6970):145-147.
Trenberth, K. E. 2003. The changing character of precipitation.
Bulletin of the American Meteorological Society 84(9):1205-1218.
Walther, G. R., E. Post, P. Convey, A. Menzel, C. Parmesan, and
et al. 2002. Ecological responses to recent climate change. Nature
416(6879):389-395.
Weyrauch, S. L. and T. C. Grubb. 2004. Patch and landscape characteristics
associated with the distribution of woodland amphibians in an agricultural
fragmented landscape: an information-theoretic approach. Biological
Conservation 115(3):443-450.
Young, B. E., K. R. Lips, J. K. Reaser, R. Ibanez, A. W. Salas,
J. R. Cedeno, L. A. Coloma, S. Ron, E. La Marca, J. R. Meyer, A.
Munoz, F. Bolanos, G. Chaves, and D. Romo. 2001. Population declines
and priorities for amphibian conservation in Latin America. Conservation
Biology 15(5):1213-1223.
|